Introduction / overview
N95 respirators and surgical masks are examples of personal protective equipment (PPE) that are used to protect the wearer from airborne particles and from fluid contaminating the face.
An N95 respirator is a respiratory protective device designed to achieve a very close facial fit and very efficient filtration of airborne particles. Note that the edges of the respirator are designed to form a seal around the nose and mouth. N95 respirators are commonly used in healthcare settings and are a subset of N95 Filtering Facepiece Respirators, often referred to as N95s.
Some N95 respirators are intended for use in a healthcare setting. Specifically, single-use, disposable respiratory protective devices are used by healthcare personnel during procedures to protect both the patient and healthcare worker from the transfer of microorganisms, bodily fluids, and particulate material.
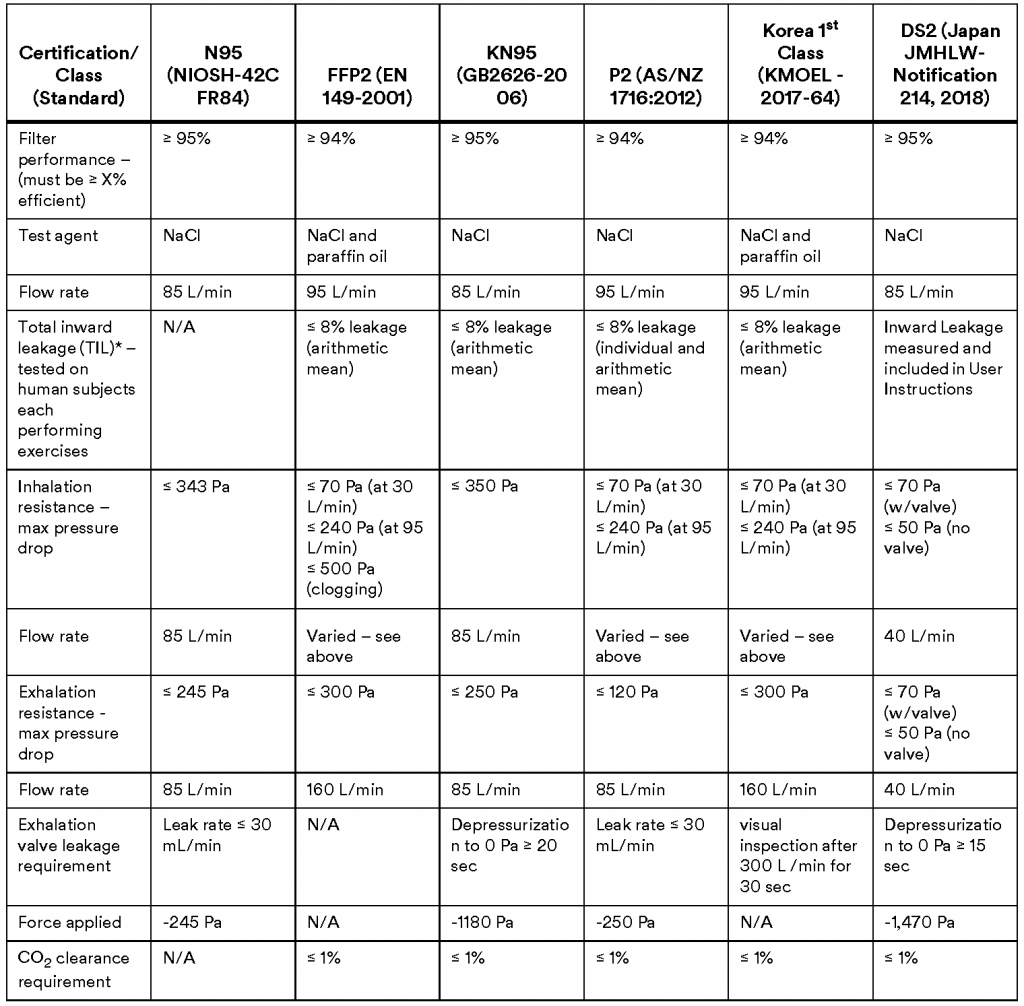
3M comparison of KN95, FFP2, and N95 filtering facepiece respirators across the world.
Filtering facepiece respirators, sometimes called disposable respirators, are subject to various regulatory standards around the world. These standards specify certain required physical properties and performance characteristics in order for respirators to claim compliance with the particular standard.
During pandemic or emergency situations, health authorities often reference these standards when making respirator recommendations, stating, for example, that certain populations should use an “N95, FFP2, or similar” respirator.
This article is intended to help clarify some key similarities between such references, specifically to the following FFR performance standards:
- N95 (United States NIOSH-42CFR84)
- FFP2 (Europe EN 149-2001)
- KN95 (China GB2626-2006)
- P2 (Australia/New Zealand AS/NZA 1716:2012)
- Korea 1st Class (Korea KMOEL – 2017-64)
- DS2 (Japan JMHLW-Notification 214, 2018)
As shown in the following summary table, respirators certified as meeting these standards can be expected to function very similarly to one another, based on the performance requirements stated in the standards and confirmed during conformity testing.